Prof. Jing-Ren Zhang’s group at Center for Infectious Disease Research, School of Medicine, Tsinghua University published research article on March 9, 2022 in Journal of Experimental Medicine entitled “Functional vulnerability of liver macrophages to capsules defines virulence of blood-borne bacteria”. This work demonstrated how the capsules enable bacterial pathogens to circumvent molecular recognition and phagocytic killing of Kupffer cells in the liver, thus define the virulence levels of blood-borne bacteria and the outcomes of septic infections.
Sepsis is a common and often lethal syndrome, which represents one of the most serious infection diseases. Encapsulated bacteria are the most common sepsis pathogens, such as Streptococcus pneumoniae, Klebsiella pneumoniae, Escherichia coli and so on. The importance of the capsule in bacterial virulence was elegantly used by Avery and his colleagues to demonstrate DNA as the genetic material in 1944, a milestone study in biology (DOI: 10.1084/jem.79.2.137). The capsule has long been regarded as slippery shield that protects bacteria from the phagocytic killing of circulating phagocytes in the blood, e.g. neutrophils, monocytes or monocyte-derived macrophages. These phagocytes are widely used in in vitro cell models. However, it remains a mystery where and how the capsules do their job in the host.
To answer this scientific question, the Jing-Ren Zhang’s group investigated the functional role of bacterial capsule in mouse sepsis model. By using multiple clinical isolates and isogenic capsule-switched derivatives of S. pneumoniae, the researchers demonstrated a decisive role of capsular type (serotype) in the virulence of pneumococci (Figure 1A). In the early phase of sepsis, the unencapsulated mutants and the low virulence (LV) capsule variants were rapidly cleared from the blood; while more than half of the inocula of the high virulence (HV) counterparts sustained in the circulation in the same period, i.e. 30 min post infection. The clearance rate of each capsular type can be ranked from the fastest to slowest as 14, 18C, 23F, 19F, 19A, 9V, 7F, 6B, 5, 1, 2, 8, 6A, 4, and 3 (Figure 1B). This parameter can be used as an objective criterion to assess the virulence of bacteria.

Figure 1. Impact of capsular types on pneumococcal virulence and bacterial clearance rate.
Further analysis revealed that the disappeared bacteria from the blood were not killed immediately but trapped in the liver. particularly, 98.7% of the LV serotype-14 pneumococci were detected in the liver as early as 5 min post inoculation. In sharp contrast, only small portion (11.6%) of the HV serotype-8 strains deposited in liver. The liver resident macrophage Kupffer cells (KCs) were the major immune cells for the rapid clearance of LV bacteria, as the depletion of KCs impaired the capture of LV strains in the liver, rendering the host more susceptible to the infection. The cellular interaction between KCs and pneumococci were lively visualized by using the intravital microscopy (IVM) technology. As exemplified in Figure 2, each KC could catch as many as 20 bacterial cells of the LV type 14 pneumococci; however, no more than one HV bacterial cell was captured by individual KC.

Figure 2. Interaction of liver KCs with capsule variants of pneumococci.
The research group next investigated how different capsules influence the immune function of KCs. Since the only variation was the capsular type, the researchers hypothesized that certain capsular polysaccharides (CPSs) action as ligands for the recognition by receptors on KCs. As expected, pre-treatment with purified CPSs blocked the early clearance of LV type 14 pneumococci in a dose-dependent manner, which was further verified by IVM observation. The CPS inhibition effect was reproducible for other LV types but not the HV capsules, indicating a direct binding of LV CPSs by KCs. Using an affinity pull down assay, the group identified the first capsule receptor on the KC, the asialoglycoprotein receptor (ASGR). After a serious of in vitro and in vivo experiments, both mouse and human ASGR can recognize serotype-7F and -14 pneumococcal capsule and promote the capture of the corresponding strains. Other LV capsules were proved to recognized by specific receptors on the KC membrane or soluble receptors in the plasma; while no binding activity was detected for the HV capsules.
Based on the findings, this work demonstrates a molecular interaction between bacterial capsule and KC receptor(s) which determines the early clearance of blood-borne bacteria and the fates of septic infections: immune clearance for the LV capsule variants or immune evasion for the HV counterparts.
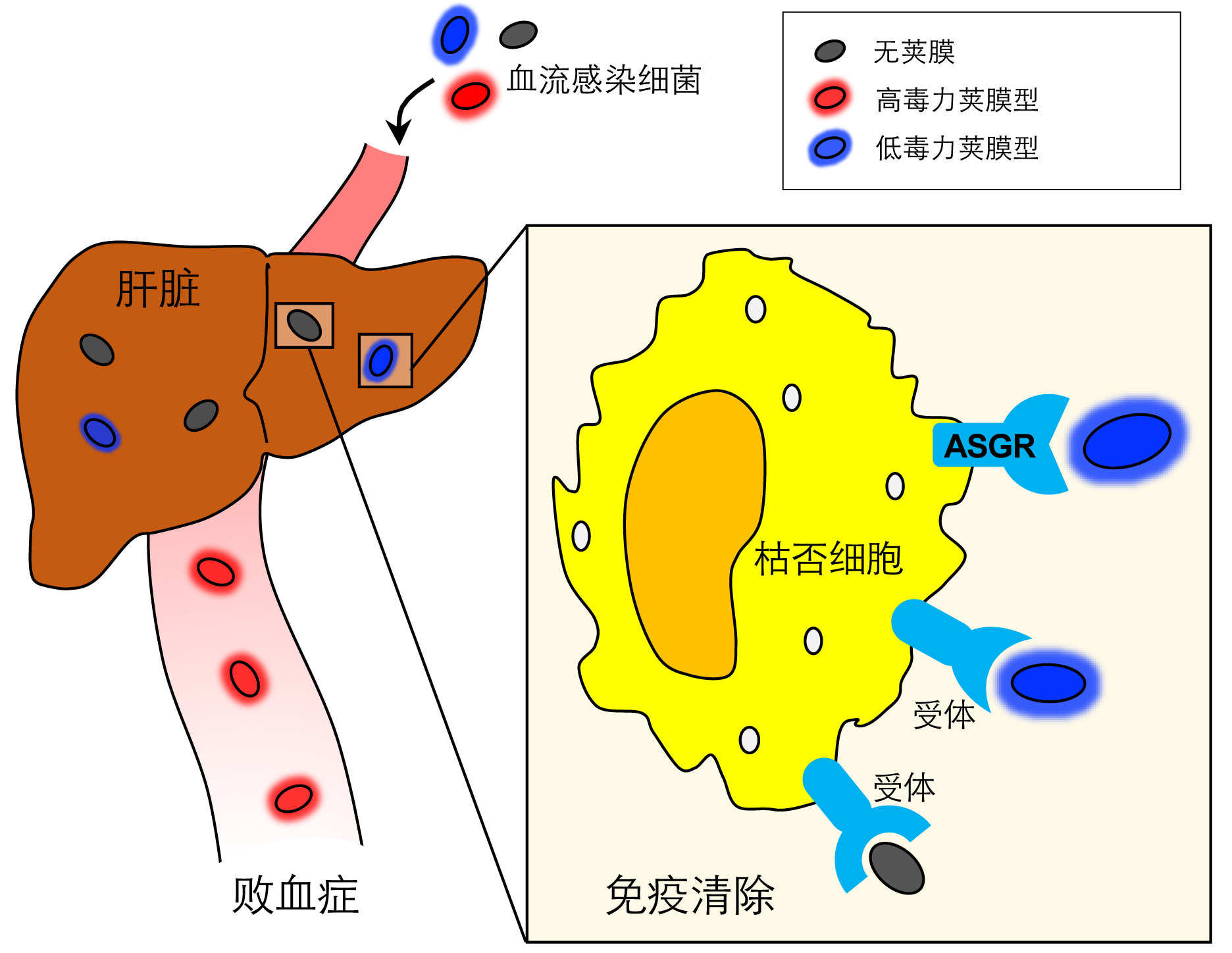
Figure 3. Capsule-receptor interaction-based molecular mechanism of sepsis.
Prof. Jing-Ren Zhang from School of Medicine, Tsinghua University is the corresponding author of this study. The group members Postdoc Haoran An, PhD student Chenyun Qian, Yijia Huang, Dr. Jing Li, and PhD student Xianbin Tian are the co-first authors. The Laboratory Animal Research Center, Center of Biomedical Analysis, Center for Cell Biology, and Center for Proteomics in Tsinghua University provided facilities for this work. This project was supported by the National Natural Science Foundation of China, Tsinghua-Peking Joint Center for Life Sciences, Tsinghua University Spring Breeze Fund, and China Postdoctoral Science Foundation.
Online Article Link: https://doi.org/10.1084/jem.20212032